5 Minute Guide to Using Disinfectants Correctly

Our world is covered in microorganisms, including bacteria, viruses, and fungi. Many of the microbes we come across daily are beneficial to us, from Lactobacillus which helps us digest dairy, to Saccharomyces cerevisiae yeast used to bake bread. However, some microbes are detrimental to our health and can cause illness if allowed to multiply. As such, various disinfectants are available to battle these pathogenic microorganisms and keep our homes clean and safe. Factors such as applied concentration, contact time and surface compatibility should be considered when selecting a suitable disinfectant.
What are disinfectants, and how do they work?
Disinfectants are chemical substances, used on non-living items to destroy microbes, including bacteria, viruses, fungi, or mold. The main active ingredient in each formula kills pathogen, usually by entering the cell and damaging it. To learn more about the kill mechanism for each active ingredient, see the table below.
There are many brands of disinfectants on the market, many of which contain similar active ingredients. Some of the most common generic disinfectants, which also happen to be in most homes, include bleach, quaternary ammonium, alcohol, and peroxides.
Common Household Disinfectants
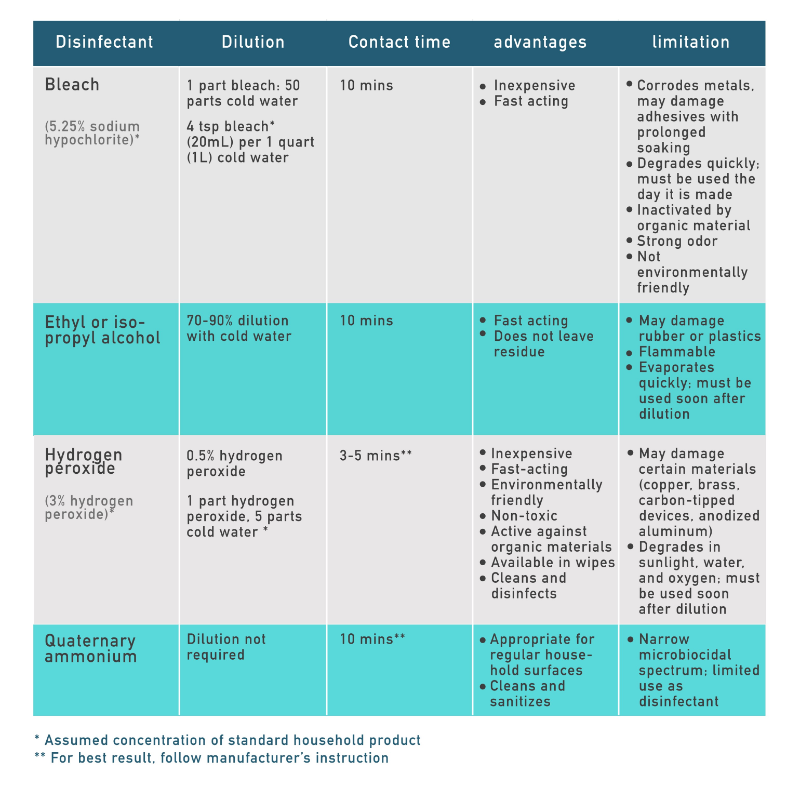
Bleach (Chlorine products)
The active ingredient in liquid bleach is either sodium hypochlorite or hypochlorous acid, while solid bleach contains calcium hypochloride or sodium dichloroisocyanurate. Bleach is a strong oxidizing agent, which causes microbe proteins to unfold and irreversibly aggregate, similar to the process which occurs when an egg is boiled. Many of these proteins are required for bacteria to grow and multiply, making bleach an effective disinfectant against a wide range of microbes.
Household bleach usually contains 5.25% sodium hypochlorite and should be diluted with cold water before use. However due to its oxidizing power, it loses potency quickly, and should used the day it is prepared. Furthermore, once bleach has been washed down the drain, it is hazardous to the environment.
Quaternary ammonium
Quaternary ammonium compounds or “quats”, are a group of compounds containing a central ammonium cation attached to 4 alkyl or aryl groups. These compounds are detergents and disinfectants and can be used to remove organic matter. Typical end-use quats have a concentration of 0.05-0.2% and have kill times of 3-10 minutes depending on the concentration. They work by interfering with bacterial cell membranes, causing cellular contents to leak, killing the cell. They also disrupt viral envelops, making them effective against enveloped viruses, as well as select fungi and amoeba.
However, quaternary ammonium compounds are not effective against endospores, mycobacterium tuberculosis, and non-enveloped viruses, and their activity is reduced by soaps and other detergents, acids, and heavy organic matter.
Alcohol
The most common alcohol disinfectants are isopropyl alcohol, and ethyl alcohol, which have germicidal characteristics. They work by disrupting bacterial cellular membranes, solubilizing lipids, and denaturing proteins by acting on protein thiol (-SH) groups, which then kills bacteria. Evidence also shows that dehydrating property of ethyl alcohol is an additional benefit, as a mixture of ethanol and water was shown to be more bactericidal than pure ethanol, as proteins denture more quickly with water.
Alcohols are also effective against fungus and lipid-containing viruses, but ineffective against spore-forming bacteria. Ethyl alcohol is most effective against bacteria when diluted to 60-90% with water, as lower concentrations of alcohol are less bactericidal, but pure ethanol evaporates too rapidly to effectively denature proteins. As a result, pure ethyl alcohol is only an effective disinfectant when an object is immersed in it.
Hydrogen peroxide
Hydrogen peroxide is an oxidizing agent and works by producing hydroxyl free radicals which damage lipids and DNA to kill bacteria, yeast viruses, spores, and fungus. Certain microbes may naturally produce catalase enzymes to protect against hydrogen peroxide by degrading it into water and oxygen, but the concentration typically used as a disinfectant is able to overwhelm this natural defense.
0.5% hydrogen peroxide can kill bacteria and viruses in 1 minute, and mycobacteria and fungi in 5 minutes. When sealed correctly, this powerful oxidizer can be stored for long periods of time, but once poured into a separate container and diluted, it degrades in the presence of sunlight, water, and oxygen.
Questions to Ask Yourself Before Choosing a Disinfectant
To choose an effective disinfectant, determine what your principle needs are, and read the product label to determine if your selected disinfectant is the right product to use. Some questions to consider include:

Bio-Vanguard antimicrobial products offer long-term 24-hour protection against a broad range of microbes including viruses, bacteria, and mold, that cause illnesses and odor. This gentle, bleach-free, and non-irritating antimicrobial is compatible with a variety of surfaces, from fabrics to plastics and stainless steel, and forms a microscopic antimicrobial protective spike bed that kills microbes for up to 30 days after application. To learn more about how Bio-Vanguard can protect your home and family against disease-causing microbes, click here, or contact us at info@bio-vanguard.com.